Objective: Students collected insects around Texas to test and see if their insects contained Wolbachia.
Objective for DNA Extraction:
The students’ objective was to extract DNA from their insect so that they can use the DNA for the polymerase chain reaction to ensure they extracted enough DNA from their insect by using the ALR elution method.
DNA Extraction Protocol:
- The insects were dissected to remove the gonads.
- A small sample was taken from the extracted gonads and put in 1.5 mL micro-centrifuge tubes.
- The tube was labeled by the student’s initials.
- 75 uL of alkaline lysis reagent was pipetted into the tubes and incubated at 65 degree Celsius for 15 minutes.
- The tubes were then put in an ice box that was 4 degree Celsius for 5 minutes.
- After the 5 minutes, 75 uL of neutralizing buffer was added in the tubes.
- They were then store in the freezer at -20 degrees Celsius.
Objective for PCR:
The students’ objective was to use the DNA extracted earlier and run a polymerase chain reaction (PCR) to ensure their insect obtained DNA by using the COI R and COI F primers.
PCR Protocol:
1. A master mix was made for 25 samples.
Master Mix for Insect DNA
| x1 | x25 | |
| Nuclease-free Water | 12.5 ul | 312.5 ul |
| 5X Buffer | 5 ul | 125 ul |
| dNTPs | 4 uL | 100 ul |
| MgCl2 | 1.75 uL | 43.8 uL |
| COI F or COI Forward Primer | 0.25 uL | 6.25 uL |
| COI R or COI Reverse Primer | 0.25 uL | 6.25 uL |
| Taq Polymerase | 0.25 uL | 6.25 uL |
2. Each PCR tube was labeled by the student’s initials.
3. 22 uL of the master mix was added into each PCR tube.
4. 3 uL of the student’s insect’s DNA was added to the tube.
5. The tube was vortex for a few seconds.
6. Then the PCR tubes were centrifuged for a few seconds so that the entire solution is at the bottom of the tube.
7. The PCR tubes were then placed into the thermal cycler and put in the “step down” program.
Thermocycler Steps
| Step | Duration | Temperature | |
| Preheat Lid | 105 degrees Celsius | ||
| 1 Cycle of Stage 1 | Initial Denaturation | 30 minutes | 94 degrees Celsius |
| 20 Cycles of Stage 2 | Denature | 30 seconds | 94 degrees Celsius |
| Anneal | 30 seconds | 56 degrees Celsius | |
| Elongation | 1 minute | 72 degrees Celsius | |
| 20 Cycles of Stage 3 | Denature | 30 seconds | 94 degrees Celsius |
| Anneal | 30 seconds | 56 degrees Celsius | |
| Elongation | 1 minute | 72 degrees Celsius | |
| Final Stage | Final Elongation | 10 Minutes | 72 degrees Celsius |
| Hold | 10 degrees Celsius |
Objective for gel electrophoresis:
Gel Electrophoresis Protocol:
1. Add .3 g of agarose to a 25 mL Erlenmeyer flask
2. Add 30 mL of 1.0x buffer and Swirl it
3. Microwave flask for 30 seconds and swirl to make sure everything is dissolved
4. Microwave flask for 30 seconds again if not clear solution
5. Add1.5 uL of ethidium bromide and swirl flask.
6. Pour the gel into gel tray and then put the comb to make wells.
7.Let the gel sit for 10-15 minutes.
8. Remove the comb and pour .25x buffer into leveling base to the fill line.
9. Use 100 base pair size standard into 1st well.
10. Add ___ uL of gel into PCR product
11. 3 uL of tube inserted to each well.
Objective for PCR:
The students’ objective was to use the DNA extraction from week 1 and run a polymerase chain reaction (PCR) to see if their insect contained the Wolbachia gene by using the Wolbachia R and Wolbachia F primers.
PCR Protocol:
- A master mix was made for 25 samples.
Master Mix for Wolbachia
| x1 | x25 | |
| Nuclease-free Water | 12.5 ul | 312.5 ul |
| 5X Buffer | 5 ul | 125 ul |
| dNTPs | 4 uL | 100 ul |
| MgCl2 | 1.75 uL | 43.8 uL |
| Wolbachia F or Wolbachia Forward Primer | 0.25 uL | 6.25 uL |
| Wolbachia R or Wolbachia Reverse Primer | 0.25 uL | 6.25 uL |
| Taq Polymerase | 0.25 uL | 6.25 uL |
3. 22 uL of the master mix was added into each PCR tube.
4. 3 uL of the student’s insect’s DNA was added to the tube.
5. The tube was vortex for a few seconds.
6. Then the PCR tubes were centrifuged for a few seconds so that the entire solution is at the bottom of the tube.
7. The PCR tubes were then placed into the thermal cycler and put in the “step down” program.
Thermocycler Steps
| Step | Duration | Temperature | |
| Preheat Lid | 105 degrees Celsius | ||
| 1 Cycle of Stage 1 | Initial Denaturation | 30 minutes | 94 degrees Celsius |
| 20 Cycles of Stage 2 | Denature | 30 seconds | 94 degrees Celsius |
| Anneal | 30 seconds | 56 degrees Celsius | |
| Elongation | 1 minute | 72 degrees Celsius | |
| 20 Cycles of Stage 3 | Denature | 30 seconds | 94 degrees Celsius |
| Anneal | 30 seconds | 56 degrees Celsius | |
| Elongation | 1 minute | 72 degrees Celsius | |
| Final Stage | Final Elongation | 10 Minutes | 72 degrees Celsius |
| Hold | 10 degrees Celsius |
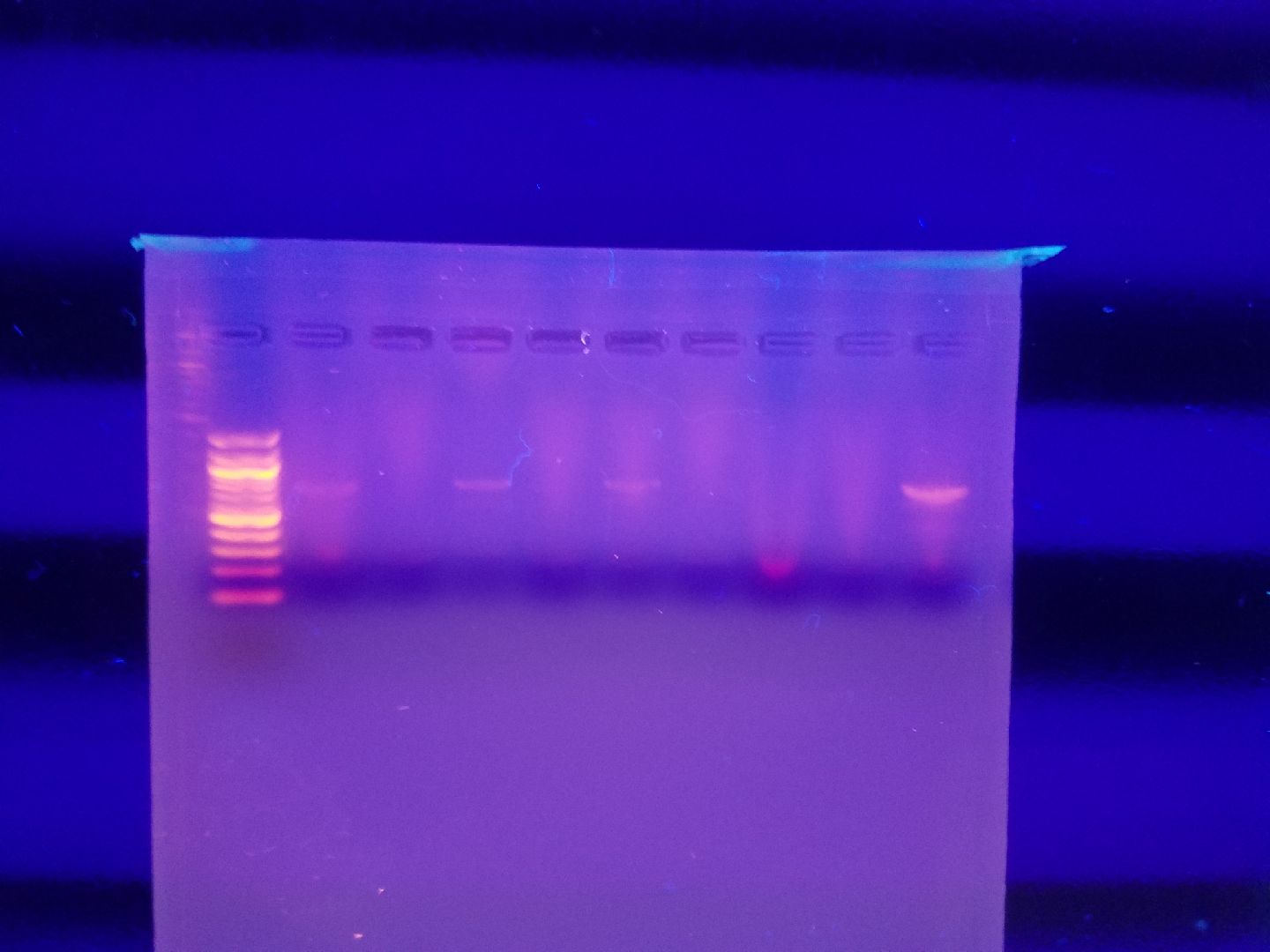
Gel Electrophoresis
*Insert results here*
Trials
Trial No.1
.
Goal: To successfully attain positive results for COI primer from PCR.
Specifications: .832 g Agarose gel.
Samples:
| Well 1: 100 bp ladder | Well 2: Spider one | Well 3: 100 bp ladder | Well 4: Spider Two | Well 5: Spider Three | Well Six: Spider Four | Well Seven: Wood Ant One | Well Eight: Wood Ant Three | Well Nine: Wood Ant Two | Well Ten: Spider Five |
| Well Eleven: 100 bp ladder | Well Twelve: Wood Ant Three | Well Thirteen: Wood Ant Four | Well Fourteen: SB1 | Well Fifteen: SB2 | Well Sixteen: SB3 | Well Seventeen: SB4 | Well Eighteen: SB5 |
Trial One results.
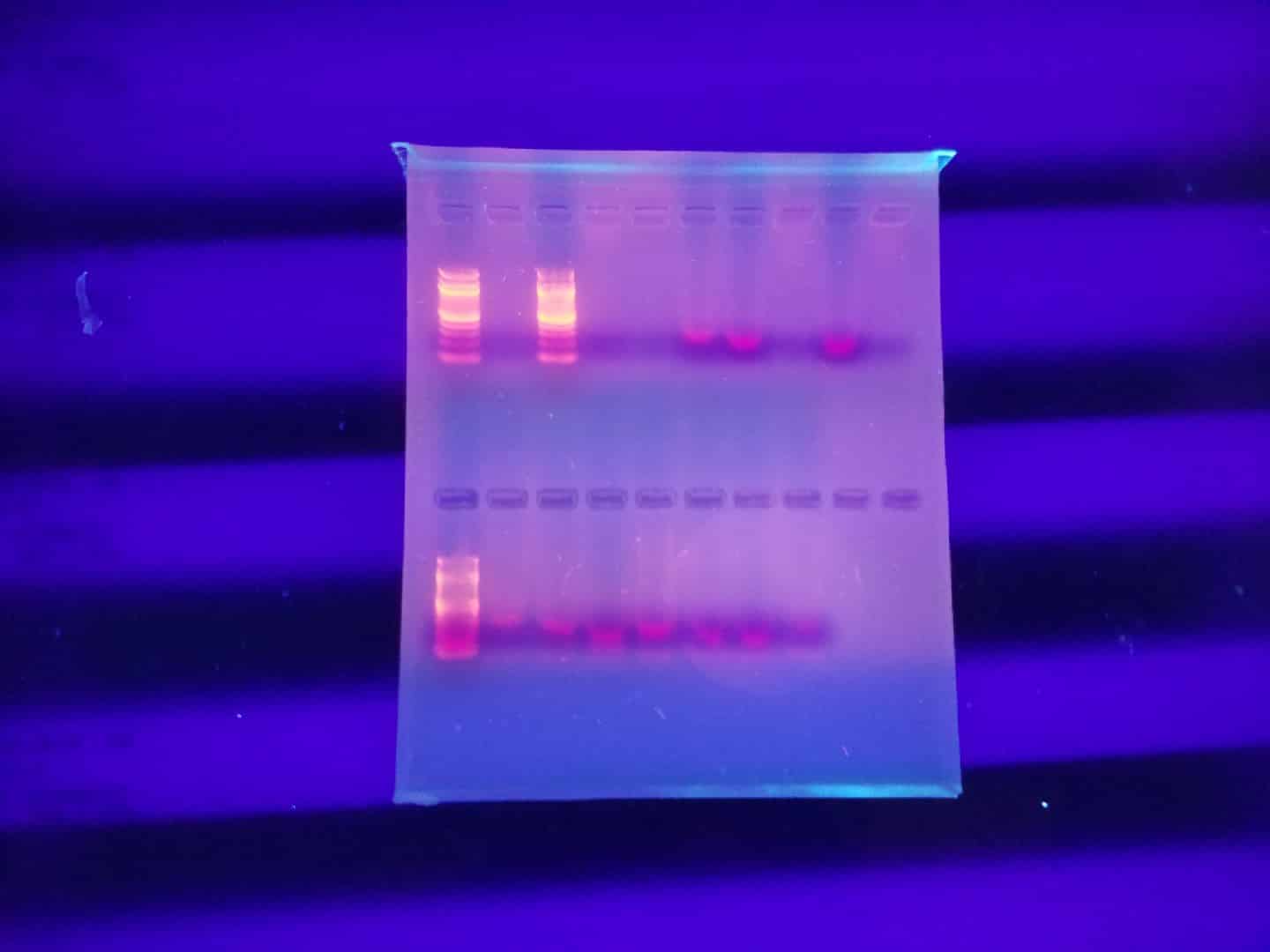
Trial No. 2
On 7/12 A second PCR was completed
Goal: To successfully attain positive results for COI primer from the designated samples.
| Well One | Well Two | Well Three | Well Four | Well Five | Well Six | Well Seven | Well Eight | Well Nine | Well Ten |
| Ladder | S1 | S2 | S3 | Sb6 | Sb7 | Sb11 | Wh6 | Wh10 | Wh11 |