Discovery of Arlo
Sample Collection
The sample was collected on September, 03, 2017 at 32°19’08.0″N 98°01’15.0″W (32.318891, -98.020827). Recorded temperature at time of collection: 91°F
September 6, 2017
Performed direct isolation on soil sample collected from Bluff Dale Community Garden. The sample was collected on September, 03, 2017 at 32°19’08.0″N 98°01’15.0″W (32.318891, -98.020827). Recorded temperature at time of collection: 91°F
- Added 7mL of soil sample to a 15mL conical
- Added 4mL enrichment broth to soil sample and incubated for 2 hours (11:45AM-1:45PM)
- Spun down sample at 4°C, 1,000 RPM, for 10min
- Plated direct isolation sample following the plaque assay protocol and incubated at 37°C initially for 24 hours
- Poor technique was used when adding top agar, preventing it from spreading across the entire plate.
- At 24 hours, one large plaque was observed. No picture was taken. Plate was returned to the incubator for an additional 24 hours to allow the plaque to grow in size and allow for additional plaques to form.
Performed enriched isolation on soil sample collected from Bosque River The sample was collected on September 6, 2017 at 32°13’22″N 98°11’55″W. Recorded temperature at time of collection: 86°F
- Followed enriched isolation protocol
- Singed the first 500uL of bacteria to be added to isolated sample and so I added 500uL more
- Let enriched isolation sample incubate with bacteria at 37°C for 48 hours
September 8, 2017
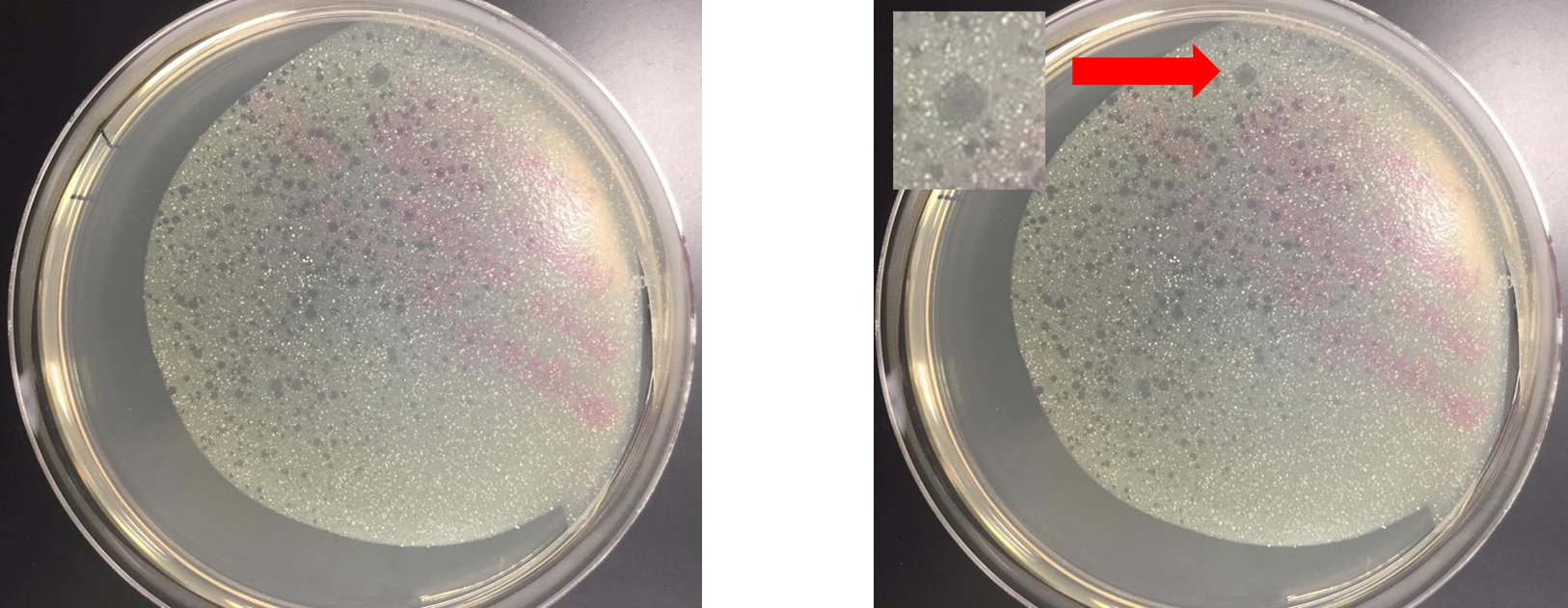
Direct Isolation plaque assay was removed from 37°C incubator at 48 hours.
- Largest plaque had formed at 24 hours and continued to grow through during the 48 hour incubation period
- Plaque exhibited a cloudy appearance, suggesting the phage has a lysogenic lifestyle
- At 48 hours, additional plaques of various sizes had appeared.
- Additional plaques were picked by Camille Trautman, Leeila Hanson, Heidi Spann and Dr. Keith Emmert to continue their studies.
September 11, 2017
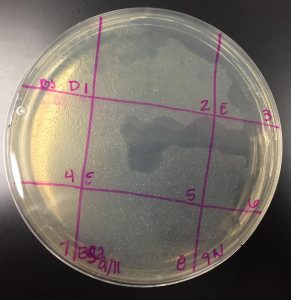
Picking a plaque
- Selected plaque was circled in teal marker and labeled “BS 9/11”
- Largest plaque present on the plate (see image above)
- Measured at: 0.3cm
- Plaque appeared at less than 24 hours and continued to grow until removed from 37°C incubator at 48 hours
- Plaque has turbid appearance, indicating that this is a temperate phage
- Largest plaque present on the plate (see image above)
- Selected additional direct isolation plaque and proceeded to spot test, but ended analysis here
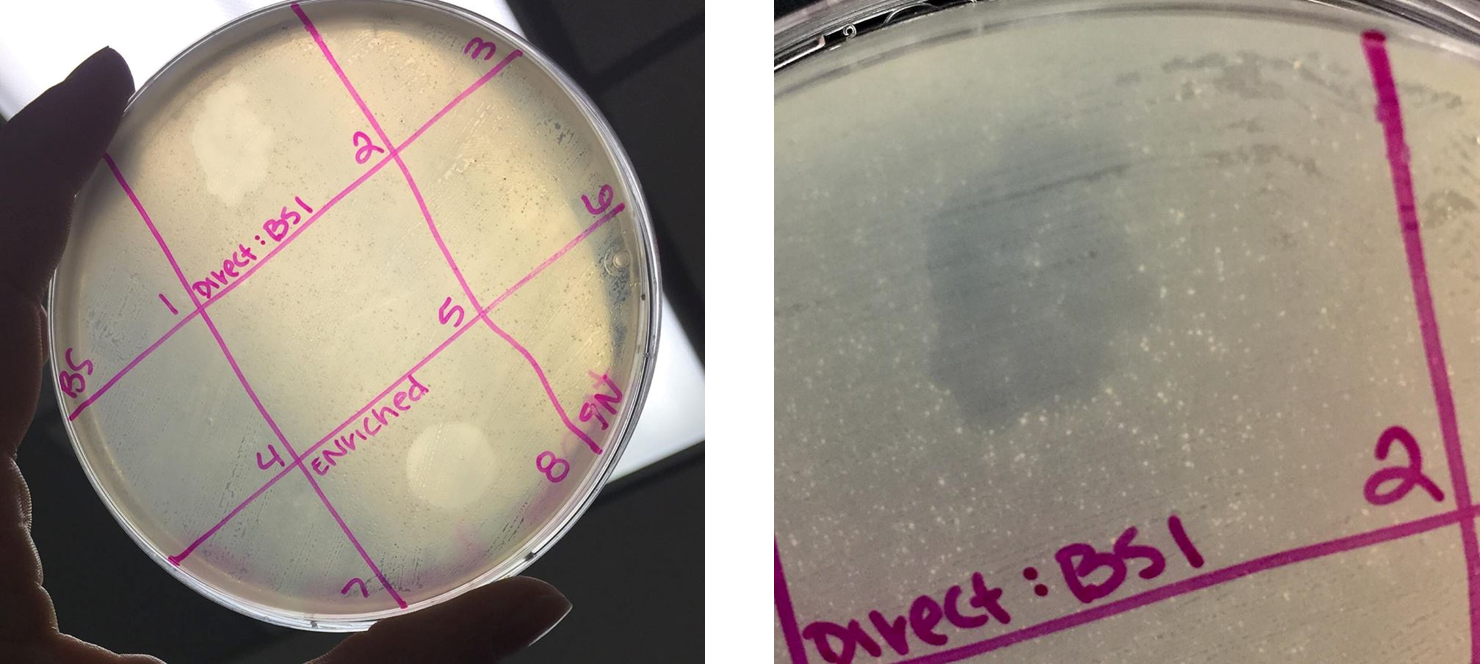
Performed spot test on both the direct isolation sample and the filtered enriched isolation sample
- Sectioned off a bacterial plate into nine sections.
- Plated a spot of 10uL of phage buffer as a negative control
- Plated a spot of 10uL of the filtered enriched sample into sections 3 and 5
- Soon after plating all samples, the plate was hit and the enriched sample smeared across the plate into sections 2 and 6 (see image below).
- Plated a spot of 10uL of the selected direct isolation sample into section 1 (labeled as “BS”)
- Bacterial clearings were observed for both direct isolation samples and the enriched isolation sample. However, due to the excessive smearing of the enriched sample, the spot test was redone.
- Plaque appeared for both direct isolation sample and enriched isolation sample.
- Direct isolation plaque came out “blotchy” with numerous spots making up a larger portion
- Enriched plaque was one large clearing
September 18, 2017
Performed serial dilutions on “BS 9/11” selected plaque from direct isolation plaque assay
- Done following the serial dilution protocol
- Selected plaque and added to 100uL of phage buffer
- Each dilution plated on a separate bacterial plate according to plaque assay protocol. More detailed notes can be found in the PDF format provided below.
- When plating, I noticed that the top agar did not solidify properly. Solidified as a single mass but did not adhere to the agar plate itself. This caused some abnormalities in my plates. Plaques still appeared on diluted plates.
- Plaques which appeared were turbid with a “target” like appearance.
- Plaques were also much larger than those obtained by other students in the class.
- These observations are consistent with the original plaque which was selected on September 11th.
- After three dilutions, only two plaques were present.
- More specific observations are included in the PDF provided below
September 20, 2017
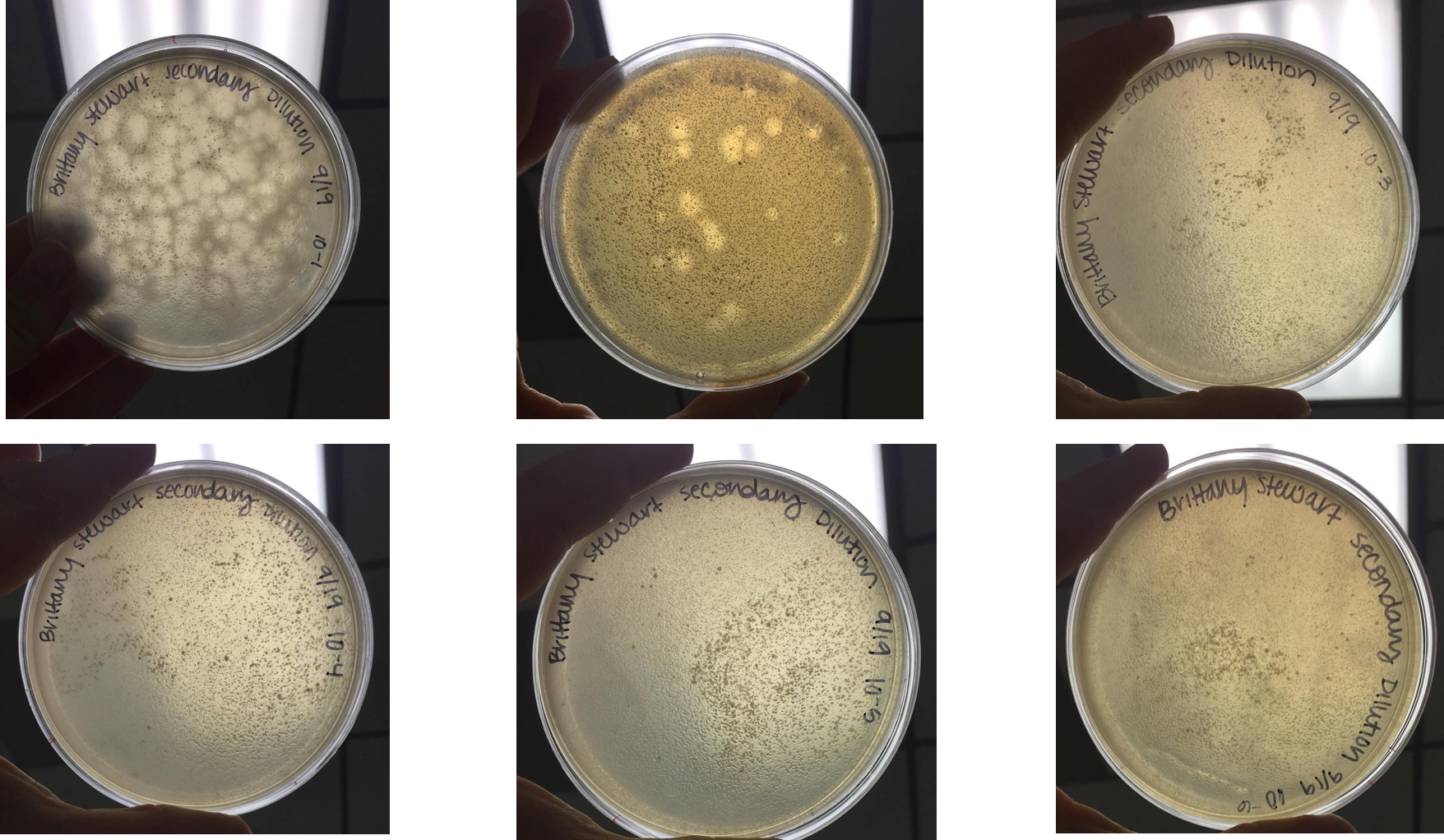
Performed second round of serial dilutions
- Selected plaque from 10(-3) plate from serial dilution performed on September 18, 2017
- Diluted this sample following the same serial dilution protocol as previously
- Incubated at 37°C for 48 hours
- At 48 hours, plaques appeared on 10(-1) and 10(-2) dilution plates.
- As previously observed, plaques were large and had a “target-like” appearance with a very turbid edge and more clear center
- 10(-1) plate contained approximately 132 plaques
- Plaques were measured to be approximately 0.5cm across
- 10(-2) plate contained approximately 21 plaques
- No further dilutions contained plaques
Number of plaques counted from secondary dilution (10^-1 dilution): Approximately 132 plaques
September 25, 2017
At 48 hours, plaques appeared on 10(-1) and 10(-2) dilution plates.
- As previously observed, plaques were large and had a “target-like” appearance with a very turbid edge and more clear center
- 10(-1) plate contained approximately 132 plaques
- Plaques were measured to be approximately 0.5cm across
- 10(-2) plate contained approximately 21 plaques
- Plaques varied from 0.5cm to 0.75cm
- No further dilutions contained plaques
Flooded plate. Allowed the plate to incubate at 4°C for 24 hours.
September 26, 2017
Collected plate lysate after incubating for 24 hours.
September 27, 2017
Performed spot titer to calculate plate titer. Let incubate 16 hours
October 4, 2017
10^-6 full plate titer showed 7 large plaques
Titer (pfu/mL)= (#pfu/volume used in uL) x (10^3 uL/mL) x 10^6
=0.8 x 10^8 pfu/mL
Medium sized plaques require 10^5 phage to make a webbed plate
Redid the full plate titer.
October 16, 2017
Making webbed plates from a lysate of known titer: Protocol 6.3
[pdf-embedder url=”https://dustinedwards.info/wp-content/uploads/2017/09/Brittany-Stewart-Phage-Isolation-Notes.pdf”]