Discovery of Astrid
INTRODUCTION
Bacteriophages have recently become one of the forefronts of modern research. Globally, their population amounts to over 10^31 particles and are highly dynamic in metabolism and genetic diversity With the resistance of bacterial superbugs becoming common clinical issue, the use of bacteriophages for antibacterial use against deadly infections has drawn the focus of the science community. Howard Hughes Medical Institutes created the Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science (SEA-PHAGES). This program aides to increase undergraduates to explore the world of bacteriophages while developing -proper scientific laboratory skills. “The Howard Hughes Medical Institute SEA-PHAGES program takes advantage of the huge size and diversity of the bacteriophage population to engage students in discovery of new viruses, genome annotation, and comparative genomics.”
BACTERIAL HOST
September 4th 2018: Protocol 5.1 Collecting Samples from the Environment
Objective: To extract bacteriophage from an environmental sample from three different locations.
Materials:
- 15mL conical tube
- small spade
- phone
- pen and paper
Procedure:
- A desired spot was located and the GPS coordinates, temperature of the air, and general type of environment in which the soil was collected in was recorded.
- Using a spade, a 6-10 inch hole was made and a sample of the soil was transferred to a 15 mL conical tube to about two-thirds full.
- The conical tube was labeled and stored immediately in the fridge until the next day until direct and enriched isolation was performed.
A total of three soil samples were collected on September 4th, 2018 from various locations in Stephenville, TX.
- Latitude: 32.21506276343063: Longitude: -98.20294943400457
- Latitude: 32.21114128379872: Longitude: -98.22142983175213
- Latitude: 32.2115830033962: Longitude: -98.2210702195454
The temperature recorded that night was 20 degrees Celsius. Location one was collected at the Stephenville City Park in a field next to a large body of water. The soil collected was a dark moist substance and was clumpy. The second location of collected soil was across from Tarleton State University, Stephenville, TX. The field was about a mile off campus and was next to a parking lot. The dirt collected here was a little more dry than the first location and was more sedimentary. The third location was about a half mile away from location two behind a storage shed. The dirt collected resembled the dirt from location one and was found near a running water supply.
September 5th 2018: Protocol 5.2 Direct Isolation of Environmental Sample
Objective: The purpose of performing a direct isolation is to successfully extract bacteriophages from the collect environment samples. In order to do this, you have to use a liquid media and filter to make sure you only get potential viral particles. Then you inoculate the host bacteria in which you want to study and plate it using Plaque Assay Protocol 5.3.
Materials:
- Environmental Sample
- Liquid Media
- Sterile 5mL syringe
- 0.22 um syringe filter
- 5ml serological pipette
- Microcentrifuge tubes
- 15ml conical tube
Procedure:
-
- The work bench was prepared using a decontaminate and working close to an open flame.
- The sample and material were then gathered and the experiment was ready to be performed.
- Extracting phage from solid environmental sample.
- A 15mL conical tube was filled about one-third full of soil.
- Liquid Media was added until the soil was submerged beneath about 2-3 mL of media.
- The tube was then capped and inverted several times to insure that the soil was mixed thoroughly.
- The tube was then shaken in a shaking incubator set at 250 rpm for 1-2 hours.
- After incubating the soil was allowed to settle in the tube for about 15 minutes.
- Preparing the Phage Filtrate.
-
- A 0.22 microliter syringe filter was used to filter out any bacteria/contaminant so that only bacteriophages go through. The package containing the filter was opened and placed on the bench with the filter still in the package. This was an important step because the filter is easily contaminated.
- Approximately 2 ml of liquid from the flooded sample was carefully removed to avoid withdrawing solid material and clogging the filter.
- The syringe was attached to the filter and was removed from the packaging.
- 0.5 ml of filtrate was dispensed in a microcentrifuge tube and labeled accordingly. The tube was capped immediately and stored in the fridge 4 degrees Celsius.
- The filter and syringe were discarded and the filtrate was ready to proceed to Plaque Assay Protocol 5.3.
September 5th 2018: Protocol 5.3 Plaque Assay
Objective: Using bacterial lawns of a selected host to detect the presence of phages. When phages are present on the bacterial lawn, there will be areas of “clearing” called plaques. Plaques let you know that viruses have replicated and lysis the bacterial in the area (area of clearing are areas that no longer have bacteria present). Each plaque may be a different phage since they arise from a single phage particle.
Materials:
- Phage sample
- Host Bacteria (250 ul/plate)
- Agar plates
- Phage Buffer
- Top Agar
- Microcentrifuge tubes
- 5 ml serological pipettes
Procedure:
-
- The workbench was prepared using aseptic technique and an open flame
- The phages from the direct isolation were gathered to be used for the plaque assay.
- Inoculating the host bacteria with the phage sample
-
- Since there was only one sample to test, one aliquot of 250 ul of host bacterial was collected and the tube was labeled accordingly.
- A micropipette was used to dispense the phage sample volume, according to the table below, into a culture tube containing 250 ul of host bacteria.
- The tube was tapped gently to mix the inoculated host culture.
- The inoculated host culture then sat undisturbed for 5-10 minutes to allow for attachment.
- After 5-10 minutes of letting the sample sit, the samples were plated using top molten agar that was provided by the instructor
- An agar plate was collected and labeled Shey Andrews; 9/05/2018:3:19PM @23C.
- A bottle of top agar was removed from the 55C bath and brought to the workbench.
- Using a 5 ml pipette, 3 ml of molten top agar was aseptically transferred into the inoculated host tube.
| Sample Type | Sample Volume |
| Direct Isolation sample | 500 ul |
| Enriched culture | 10 ul |
| Serial Dilutions of picked plaques | 10 ul |
| Lysates for titering | 10 ul |
| Negative Control | 10 ul of phage buffer |
| Positive control | 10 ul provided phage sample |
-
- The molten top agar/inoculated host mix was then immediately aspirated back into the pipette and was transferred to the agar plate.
- The agar plate was quickly and gently tilted so that the top agar mixture evenly coated the plate.
- Incubating the plates
- The plates were incubated to allow for bacterial growth and phage infection.
- The top agar was allowed to solidify by letting the plates sit for approximately 20 minutes undisturbed.
- The plates were inverted and placed in the incubator for 24-48 hours at 24-30 C.
September 7th 2018: Protocol 5.3 Results from 09/06/2018 Plaque Assay
The plate that was put in the incubator on 09/06/2018 shows no plaques. This means that there was no phage particles successfully transferred to the host and plate. Also the top agar did not set properly. The distillate from the direct isolation used in this plaque assay was used as a duplicate and the sample was tested again for the presence of phage.
September 7th 2018: Protocol 5.3 Plaque Assay Duplicate
Objective: To extract bacteriophage from the direct isolation distillate.
Materials: same as Protocol 5.3 Plaque Assay on September 5th.
Procedure:
- Modifications
- The procedure was the same as the Plaque Assay performed on September 5th with a few modifications.
- When pipetting the extracted phage from the centrifuge tube to the bacterial host test tube, extra care was taken to make sure that the phage sample made it completely into the host by avoiding the sides of the test tube.
- Before inoculating the host, the tube was flicked and observed to make sure that the culture was as homogenous as it could get and after adding the phage it was mixed again by flicking it.
- The attachment time was also physically timed to 20 minutes to make sure the phage was properly attached.
- The inoculated culture was then plated using the same steps in the previous run of protocol 5.3.
- The procedure was the same as the Plaque Assay performed on September 5th with a few modifications.
September 10th 2018: Protocol 5.3 Results from Plaque Assay on 09/07/2018
Results: This photo was taken on September 10th 2018. This is the plate that was incubated from 09-07-2018 and was from the same sample of direct isolation. There were no plaques present on this plate which meant that there were no phages present. Our next step was to perform an enriched isolation on another soil sample.
September 11th 2018: Protocol 5.5 Enriched Isolation of Soil Sample from Collection Site Two
Objective: The objective of doing an enriched isolation is to amplify the amount of phage present in the environmental sample. The result of doing the incubation of the soil sample is a culture with an exponentially larger in concentration of phages that is specific to the bacterial host.
Materials:
- Solid Environmental Sample
- 0.22 microliter syringe filter
- Liquid Media
- Microcentrifuge tubes
- 500 microliters of host bacteria
- 50 mL sterile conical tube
Procedure:
-
- The solid environmental sample was gathers along with the materials.
- Extracting the Phage from the soil sample
-
-
- A 50 mL conical tube was filled with the soil to the 15 mL mark.
- Liquid media was added to the 35 mL mark and the tube was vortexed.
- The sample was then placed in the shaking incubator for 1-2 hours at 250 rpm.
- After settling, the tube was placed and balanced inside the centrifuge machine and was centrifuged at 2,000xg for 10 minutes in order to pellet the soil.
-
- Preparing Bench and Seeding the Culture with Host Bacteria
-
- The supernatant was filtered through a 0.22 microliter filter to remove unwanted bacteria and soil particles.
- The volume of supernatant collected was about 15 mL.
- 0.5 mL of bacterial host culture was added to the conical tube.
- The cap was placed on the conical tube. Care was taken to make sure that the cap was properly aerated. This was done by loosely placing the cap and securing it with a piece of tape.
- The conical tube was incubated in the shaking incubator (upright to avoid spillage) for 2-5 days at 220 rpm.
- After the enriched culture incubated for two days, the next step was done.
- 1.4 mL of the enriched culture was transferred using a pipette into a microcentrifuge tube. This step was repeated twice so that there are two microcentrifuge tubes with 1.4 mL in each.
- The tubes were spun at high speed in a microcentrifuge for 1 minute to pellet the bacteria.
- The supernatant was not clear so the following was performed:
- The plunger from the syringe was removed
- The sterile filter was opened and attached to the barrel of the syringe
- 1 mL of supernatant was pipetted from each of the microcentrifuge tubes into the syringe barrel for a total of about 2 mL.
- The tip of the syringe (that was attached to the filter) was placed over the microcentrifuge tube and the plunger was inserted to the syringe and depressed.
- The microcentrifuge tube was centrifuged for at high speed for 1 minute to ensure that the bacteria is pelleted.
- The supernatant what transferred into a clean microcentrifuge tube and capped immediately.
- The next step that was performed was the Spot Test Protocol 5.6
September 10th 2018: Protocol 5.6 Spot Test on Enriched Isolation
Objective: To test a sample for the presence of phage on a bacterial lawn by “spotting” the plate.
Materials:
- Liquid phage sample from enriched isolation
- Agar plates
- 250 ul of host bacteria
- To agar
- Phage Buffer 5mL serological pipette
Procedure:
-
- The bench was prepared for aseptic work and the supplies and liquid phage from the enriched isolation was gathered.
- Preparing Bacterial Lawn Using Aseptic Technique
-
-
- The agar plate was labeled into three sections (AR1, AR2, SA1).
- 3 mL of molten top agar (provided by the instructor) was transferred into a 250 ul culture of host bacteria using a sterile 5 mL pipette.
- The solution was immediately drawn from the tube and dispensed onto an agar plate.
- The agar plate was gently and quickly tilted in multiple directions until the agar evenly coated the plate.
- The plate sat undisturbed for 20 minutes and the top agar solidified completely.
-
- Spotting the Liquid Phage on the prepared bacterial lawn.
-
- 10 ul of each sample was used to spot the bacterial lawn one at a time.
- The tip was held slightly above the agar and expelled so that the drop did not splatter.
- The spots were placed in a triangle pattern on each of the three sections of the plate.
- Without inverting the plate, it was incubated at 24-30C for 24-48 hours.
September 12th 2018: Results from Spot Test of Enriched Isolation
This image was taken on 09/12/2018. The results show that there are no plaques on the plate so none of the samples from the enriched isolation contained phage. There is evidence that there was some bacterial contamination (yellow areas on the plate). This most likely resulted from a cross contamination between the bacterial host and the phage during transfer. A second spot test was performed using the same liquid phage sample from the enriched isolation. During the second spot test, a phage buffer was added as a negative control.
September 15th 2018: Protocol 5.6 Spot Test on Enriched Isolation
Objective: Test sample for the presence of phage.
Materials: Same as before with Protocol 5.6.
Procedure:
- Modifications
- The only modification that was performed was to the labeling of the agar plate. The agar plate this run was sectioned into four different sections (Phage Buffer, AR1, AR2, SA1). A phage buffer was added as a negative control. The samples that were collected from the enriched isolation used in the previous spot test was also used during this run of the experiment.
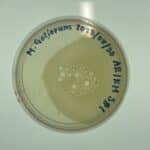
September 17th 2018: Protocol 5.6 Results from Spot Test 2
The plate was incubated for two days. There were no plaques observed on 09/17/2018 when this picture was taken which means that there are no phage present in the sample.