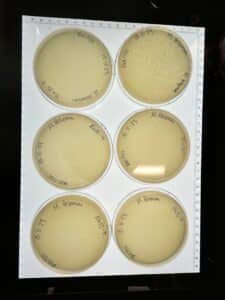
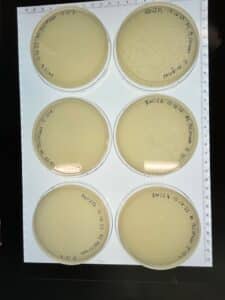
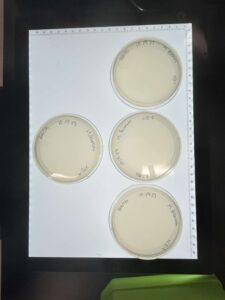
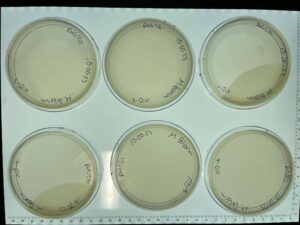
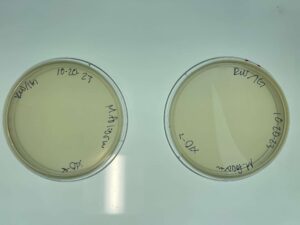
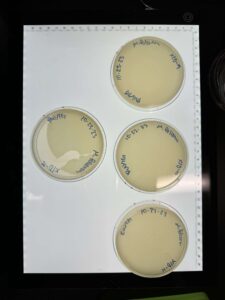
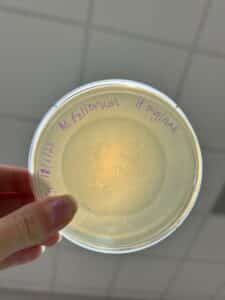
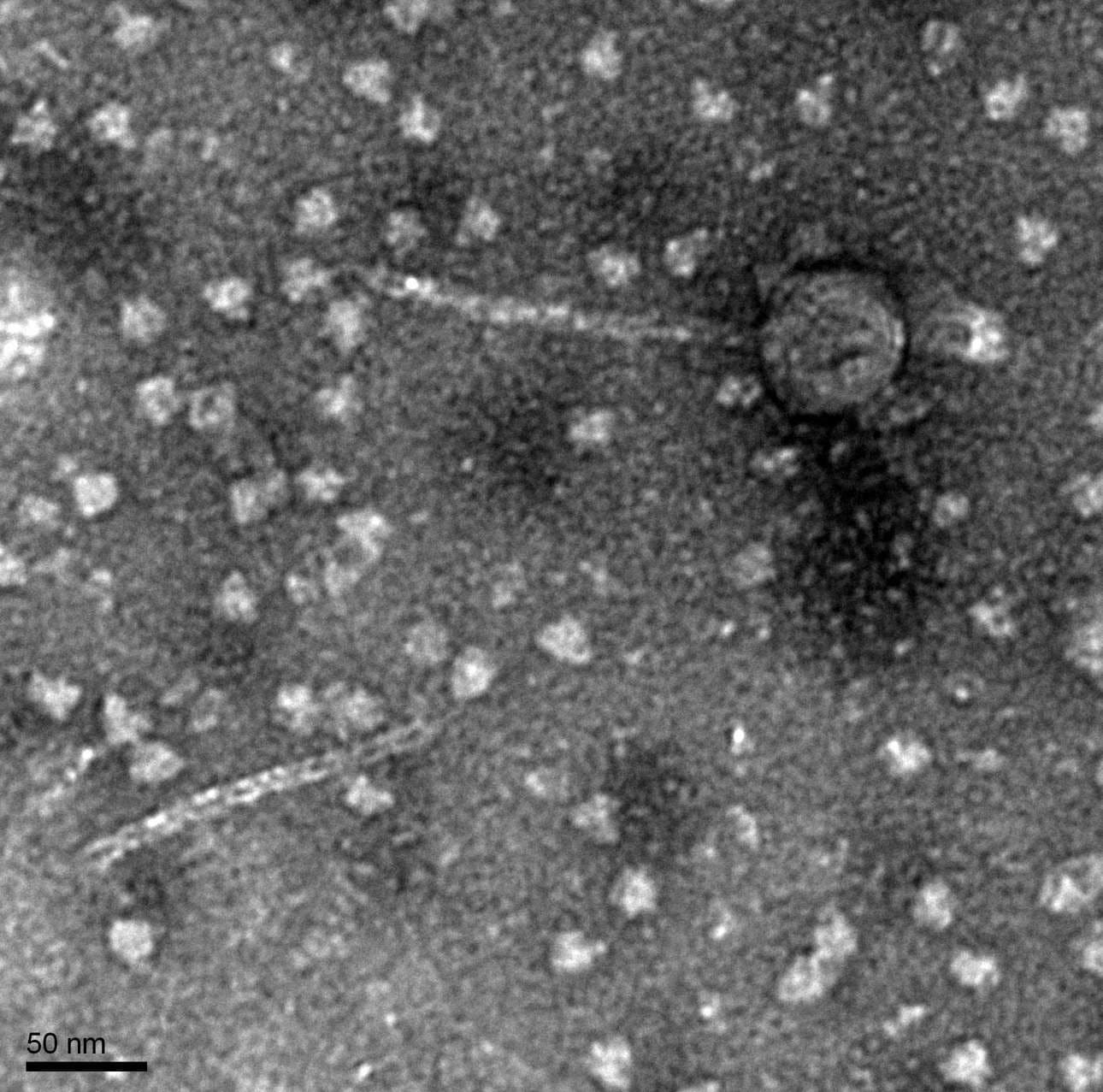
Discovery of BoneCarver
BoneCarver Information
Morphology: Siphoviridae
Sample Collection
| Collector Name |
Rylee Widger | Isabel Gonzaba | Rylee Widger | Isabel Gonzaba | Rylee Widger |
| Sample No. | 1 | 2 | 3 | 4 | 5 |
| Date of Collection | 08/29/23 | 08/29/23 | 09/06/23 | 09/11/23 | 09/13/23 |
| Sample Type | Soil | Soil | soil | soil | soil |
| General Location | Stephenville, TX | Stephenville, TX | Stephenville, TX | Stephenville, TX | Stephenville, TX |
| Location Description | The edge of a neglected flowerbed on W. Green St. | 5 feet away from porch in backyard on Harbin Dr. | 10 feet out of the door of the science building of the Tarleton campus | next to a trashcan outside of Legends dorm | Beside a small bench in the dog park on the Tarleton campus |
| GPS Coordinates | 32.21945 °N, 98.20829 °W | 32.21326 °N, 98.22464 °W | 32.21710 °N, 98.21997 °W |
32°13’3″ N 98°13’15” W
|
32°13’8″ N 98°13’6″ W |
| Sample Depth | 1.5 — 2 in. | 1.5 — 2 in. | 1 in. | 2 — 2.5 in | 2.5 — 3 in |
| Ambient Temperature | 29.4°C | 29.4°C | 22.8°C | 21.1°C | 20.5°C |
| Collector Name |
Isabel Gonzaba | Isabel Gonzaba | Rylee Widger | Rylee Widger | Rylee Widger |
| Sample No. | 6 | 7 | 8 | 9 | 10 |
| Date of Collection | 09/13/23 | 09/18/23 | 09/28/23 | 09/28/23 | 09/28/23 |
| Sample Type | soil | soil | soil | soil | soil |
| General Location | Stephenville, TX | Stephenville, TX | Stephenville, TX | Stephenville, TX | Stephenville, TX |
| Location Description | Under a bush on the side of Heritage dorm | In a questionable corner in my backyard | On the side of a trail | Under a lightpost on a trail | A ditch in a field |
| GPS Coordinates |
32°13’5″ N 98°13’9″ W |
32.21326 °N, 98.22464 °W | 33°1’25″N, 97°13’22″W | 33°1’21″N, 97°13’22″W | 33°1’29″N, 97°13’24″W |
| Sample Depth | 1.5 in | 1.5 in | 2 in | 2 in | 2 in |
| Ambient Temperature | 20.5°C | 21.1°C | 14°C | 14°C | 14°C |
Isolation/Purification
Title: Isolation/Purification of Environmental Sample
Date: 08/30/23
Redo: No
Sample: 1
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble supplies.
- Collect an environmental sample using proper collection techniques.
- Extract phage from solid environmental samples, such as soil or compost.
- Fill a 15 ml tube about one-third to one-half full with soil.
- Add liquid media until the sample is submerged beneath 2–3 ml of liquid.
- Cap the tube and invert several times to mix.
- Incubate the tube in a shaking incubator at 250 rpm for 1–2 hours.
- Allow the sample to sit until particulate matter has mostly settled.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. If your filter clogs, remove the clogged filter, replace it with a new one, and continue.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging.Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package.
- Dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
Petri dish was contaminated.
Conclusions and Next Steps:
Retrieve new sample and proceed with enriched isolation.
Title: Isolation/Purification of Environmental Sample by Plaque Assay
Date: 08/30/23
Redo: No
Sample: 2
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble your supplies.
- Collect an environmental sample using proper collection techniques.
- Extract phage from solid environmental samples, such as soil or compost.
- Fill a 15 ml tube about one-third to one-half full with soil.
- Add liquid media until the sample is submerged beneath 2–3 ml of liquid.
- Cap the tube and invert several times to mix.
- Incubate the tube in a shaking incubator at 250 rpm for 1–2 hours.
- Allow the sample to sit until particulate matter has mostly settled.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. If your filter clogs replace it with a new one, and continue filtering.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging. Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package.
- Dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
Petri dish was contaminated.
Conclusions and Next Steps:
Retrieve new sample and proceed with enriched isolation.
Title: Isolation/Purification of Environmental Sample
Date: 09/06/23
Redo: Yes
Sample: 3
Purpose: Isolate and amplify phage with enriched isolation techniques.
Notes:
- Extract phages from a soil sample.
- Using a 15ml conical tube, fill it to about ⅓ – ½ full with soil for sample collection.
- Add soil sample to a 50ml conical tube to the 15ml mark.
- Add liquid media to the 35ml mark and vortex at ~250rpm for 1–2 hours.
- Balance the tubes and centrifuge at 2,000 x g for 10 minutes to force most of the soil to the bottom of the tube.
- Prepare the bench for aseptic work and assemble supplies.
- Filter the supernatant through a 0.22 µm filter to remove unwanted bacteria and soil particles
- Collect the flow through in a sterile baffled Erlenmeyer flask or a 50ml sterile conical tube
- Recovered volumes will range between 20 and 25 ml.
- Seed the culture with host bacteria.
- Add 0.5ml of bacterial host culture to the flask or conical tube.
- Incubate the flask or conical tube at the proper temperature, shaking at 220rpm for 2-5 days.
- If you are using a 50ml conical tube, you must ensure that the culture will be properly aerated. To do so, screw the cap on one-quarter of a turn so that the conical tube is only loosely capped, and then secure the cap with a short piece of lab tape to ensure it does not fall off. Check to make sure that the conical tube remains only loosely capped. Tubes must remain upright while being shaken, and care taken to avoid spillage.
- Filter the enriched culture.
- Using an appropriate pipette, transfer 1.4ml of your enriched culture from the Erlenmeyer flask to a microcentrifuge tube.
- Repeat this procedure so that you have two microcentrifuge tubes, each with 1.4ml of enriched culture.
- Spin the tubes at high speed in the microcentrifuge for 1 minute to pellet the bacteria.
- If your supernatant is not clear or if you suspect your enrichment contains non-host bacteria, filter the supernatant through a 0.22µm filter as described below. Otherwise, proceed directly to step 5.
- Remove the plunger from a syringe.
- Open a sterile filter and attach it to the barrel of the syringe.
- Pipette 1ml of supernatant from each microcentrifuge tube into the syringe barrel (for a total of 2ml).
- Place the tip of the filter/syringe over a sterile microcentrifuge tube and insert the plunger into the syringe.
- Depress the plunger and collect the sterile filtrate.
- Transfer the supernatant into a clean microcentrifuge tube, avoiding the bacterial pellet.
- Immediately cap the microfuge tube containing your supernatant or filtrate and label is appropriately. Should be stored at 4°C.
- Either return your culture to the incubator, or dispose of you enriched culture as directed by your instructor.
- As directed by your instructor, your next step will be to test your supernatant for phages by using a Spot Test.
Results:
Unclear result.
Conclusions and Next Steps:
Filter the sample before plating.
No plaque found on the petri dish after plating; retrieve a new sample.
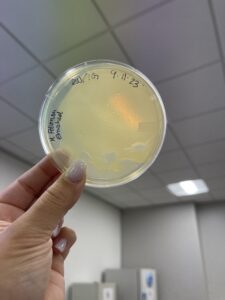
Title: Isolation/Purification of Environmental Sample
Date: 09/11/23
Redo: Yes
Sample: 4
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble your supplies.
- Collect an environmental sample using proper collection techniques.
- Extract phage from solid environmental samples, such as soil or compost.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. If your filter clogs replace it with a new one, and continue filtering.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging.Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package.
- Dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
No plaque found on petri dish.
Conclusions and Next Steps:
Collect new sample and start over.
Title: Isolation/Purification of Environmental Sample
Date: 09/13/23
Redo: Yes
Sample: 5
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble your supplies.
- You will need an environmental sample collected using the protocol Collecting Environmental Samples.
- Extract phage from solid environmental samples, such as soil or compost.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. Do not continue to force liquid through the filter or it will break. If your filter clogs, remove the clogged filter, replace it with a new one, and continue filtering.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging.Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package. Be careful not to contaminate the filter in the process.
- Depressing the syringe plunger, dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
No plaque found on petri dish.
Conclusions and Next Steps:
Collect a new sample and start over.
Title: Isolation/Purification of Environmental Sample
Date: 09/13/23
Redo: Yes
Sample: 6
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble your supplies.
- Collect an environmental sample using correct collection techniques
- Extract phage from solid environmental samples, such as soil or compost.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. If your filter clogs replace it with a new one, and continue filtering.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging. Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package.
- Dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
No plaque found on petri dish.
Conclusions and Next Steps:
Collect new sample and start over.
Title: Isolation/Purification of Environmental Sample
Date: 09/18/23
Redo: Yes
Sample: 7
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble your supplies.
- Collect a sample using correct collecting techniques.
- Extract phage from solid environmental samples, such as soil or compost.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. If your filter clogs replace it with a new one, and continue filtering.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging. Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package.
- Dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
Some plaque may be found.
Conclusions and Next Steps:
Take out the possible plaque and try to replicate.
Title: Isolation/Purification of Environmental Sample
Date: 09/20/23
Redo: Yes
Sample: 8
Purpose: Isolate a bacteriophage from a soil sample collected from the environment, and add it to a host bacteria. This mixture is added to a petri dish to identify plaque formations.
Notes:
- Prepare your bench for aseptic work and assemble your supplies.
- Collect a sample using correct collecting techniques.
- Extract phage from solid environmental samples, such as soil or compost.
- Prepare a phage filtrate using aseptic technique.
- Avoid withdrawing any solid material from the bottom of the tube to prevent clogging the filter during filtration.
- Make sure the filter is screwed firmly into place.
- Because debris can clog the filter, you may encounter resistance. If your filter clogs replace it with a new one, and continue filtering.
- Cap the tube immediately.
- Open the package of a syringe filter (0.22 μm), leaving the filter in the packaging. Using a syringe, remove approximately 2 ml of liquid from the top of the flooded sample.
- Attach the syringe to the top of the filter, and then remove the filter from the package.
- Dispense a minimum of 0.5 ml of filtrate into a labeled microcentrifuge tube.
- Discard the syringe and filter.
Results:
XX.
Conclusions and Next Steps:
XX.
Amplification
Title: First Round of Serial Dilution
Date: 09/20/23 Redo: No Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
-
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
- Pick an isolated plaque.
Results:
Unclear plates.
Conclusions and Next Steps:
Return to original sample and redo first round of serial dilution.
Title: First Round of Serial Dilution
Date: 9/25/23 Redo: Yes Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Series of diluted and isolated plaques.
Conclusions and Next Steps:
Plaques were isolated successfully, proceed to second serial dilution.
Title: Second Round of Serial Dilution
Date: 9/27/23 Redo: Yes Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Unclear plates.
Conclusions and Next Steps:
Return to original sample and redo first round of serial dilutions.
Title: First Round of Serial Dilution
Date: 10/2/23 Redo: Yes Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Only the original and 10-1 plates had plaque.
Conclusions and Next Steps:
Pick a plaque on the plate and proceed to second serial dilution.
Title: Second Round of Serial Dilution
Date: 10/4/23 Redo: No Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
No plaques found on any plates.
Conclusions and Next Steps:
Go back to the original plate and test one of the other plaques and proceed from there.
Title: Test Serial Dilution
Date: 10/9/23 Redo: No Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Plaques found on both original and 10-1 tested plates.
Conclusions and Next Steps:
Pick a plaque from one plate and continue on with first round of serial dilution.
Title: First Round of Serial Dilution
Date: 10/11/23 Redo: No Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Only the original and 10-1 plates had plaque on them.
Conclusions and Next Steps:
Pick a plaque from the 10-1 plate and proceed to the second serial dilution.
Title: Second Round of Serial Dilution
Date: 10/16/23 Redo: No Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Success growth of plaques.
Conclusions and Next Steps:
Proceed to flooding plaques.
Title: Collecting Plate Lysates
Date: 10/18/23 Redo: no Sample: 7
Purpose: To generate a highly concentrated liquid phage sample
Notes:
A. Identify one or more plates for lysate collection. Photograph and label the plate in notebook.
B. Flood the webbed plate(s).
-
- Apply 8 ml of sterile phage buffer to the webbed plate.
- Let the plate(s) sit at room temperature for 2–4 hours.
- Swirl the phage buffer gently, taking care not to splash.
C. Harvest a plate lysate.
-
- When the incubation time is complete, remove the lid from the plate and place it on the bench. Tilt the plate slightly by placing one edge of the plate on the lid, allowing the lysate to pool to one side.
- Prepare a 0.22 μm filter by opening the packaging but not removing the filter. Set aside.
- Using a 5 ml syringe aspirate (suck up) the lysate from the plate.
- Carefully attach the syringe to the filter. Depress the syringe plunger and collect the filtrate in a 15 ml sterile conical tube.
- If you still have unfiltered lysate remaining on your plate, remove the filter and store it in its plastic packaging to maintain sterility. Aspirate the remaining lysate, reattach the used filter, and filter the remaining lysate, collecting the filtrate in the same sterile conical tube.
- Label the tube appropriately.
D. Pool the lysates.
-
- If you have multiple webbed plates for a single phage sample, the same filter can be used for all plates if it remains sterile and unclogged. However, if it has been contaminated or becomes clogged, use a new filter.
- Combine all of the filtered lysates into the same sterile conical tube.
- Record the final volume of lysate collected.
Results:
After 2-4 hours, lysate was successfully collected.
Conclusions and Next Steps:
Plate the first four plates of lysate and proceed.
Title: Full Plate Titer
Date: 10/19/23 Redo: No Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-6
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
All four plates were cleared out.
Conclusions and Next Steps:
Create more dilutions up to 10-8.
Title: Full Plate Titer
Date: 10/20/23 Redo: yes Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-8
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
All eight plates were cleared out.
Conclusions and Next Steps:
Create more dilutions up to 10-12.
Title: Full Plate Titer
Date: 10/23/23 Redo: yes Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-12
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
No bacteria was cleared.
Conclusions and Next Steps:
Go back to 10-8 dilutions.
Title: Full Plate Titer
Date: 10/24/23 Redo: yes Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-8
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
Bacteria cleared out up to 10-8 dilutions.
Conclusions and Next Steps:
Do up to 10-5 dilutions with a negative control.
Title: Full Plate Titer
Date: 10/25/23 Redo: yes Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-8
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
No plaques formed.
Conclusions and Next Steps:
Go back to the original plate and pick a new plaque.
Title: First Round of Serial Dilution
Date: 11/1/23 Redo: Yes Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Plaques were successfully isolated.
Conclusions and Next Steps:
Pick another plaque and proceed to second serial dilution.
Title: Second Round of Serial Dilution
Date: 11/2/23 Redo: No Sample: 7
Purpose: To generate well-isolated plaques.
Notes:
- Pick an isolated plaque.
- Draw a circle around the isolated plaque on the bottom of the plate and label it. If there is more than one plaque, label each plaque something different.
- Using aseptic technique, aliquot 100μl of phage buffer into each microcentrifuge tube.
- Place a sterile tip onto a p200 micropipettor.
- Holding the pipettor perpendicular to the agar surface, gently stab the top agar in the center of the plaque and avoid touching the surrounding bacteria.
- Place the end of the tip into the phage buffer in the corresponding microcentrifuge tube, then tap the tip on the wall of the tube and pipette up and down to dislodge phage particles. Discard tip.
- Mix by vortexing.
- Repeat steps c-f for each plaque you are picking.
- 10-fold serial dilution of selected plaque.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-5.
- Add 90μl of phage buffer to each of the tubes.
- Add 10μl of your undiluted phage sample to the “10-1” tube and shake well.
- Transfer 10μl of the “10-1” sample to the “10-2” tube and shake well.
- Continue each dilution until you get to your last tube.
- Innoculate the host bacteria with phage sample.
- Obtain the same number of aliquots of 250μl host bacterial cultures as you have the phage samples. Label the tubes accordingly.
- Use a micropipettor and aseptic technique to add 10μl of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
- Let the sample sit undisturbed for 5-10 minutes to allow for attachment.
- Plate the samples with top agar. You will need 3ml of molten top agar per sample.
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55°C bath. Keeping the top agar in the 55°C for as long as possible will help the agar from prematurely solidifying on your work bench.
- For each sample:
- Using a sterile 5ml pipette, aseptically transfer 3ml of top agar to an inoculated host tube (the tube containing bacterial host and phage sample). Try to avoid making or withdrawing bubbles, as they can end up looking like plaques on plates.
- Immediately suck the mixture back up into the pipette and transfer it to the appropriate plate is discard the pipette. The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify.
- Gently but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Repeat this process for each of your samples.
- Incubate plates to allow for bacterial growth and phage infection.
- Let the plates sit undisturbed from about 20 minutes until the top agar solidifies.
- After the top agar has solidified, gently invert the plates and place in the proper incubator.
- Incubate the plates at the specified temperature for 24-48 hours.
Results:
Successful growth of plaques.
Conclusions and Next Steps:
Proceed to flooding plaques.
Title: Collecting Plate Lysates
Date: 11/3/23 Redo: no Sample: 7
Purpose: To generate a highly concentrated liquid phage sample
Notes:
A. Identify one or more plates for lysate collection. Photograph and label the plate in notebook.
B. Flood the webbed plate(s).
-
- Apply 8 ml of sterile phage buffer to the webbed plate.
- Let the plate(s) sit at room temperature for 2–4 hours.
- Swirl the phage buffer gently, taking care not to splash.
C. Harvest a plate lysate.
-
- When the incubation time is complete, remove the lid from the plate and place it on the bench. Tilt the plate slightly by placing one edge of the plate on the lid, allowing the lysate to pool to one side.
- Prepare a 0.22 μm filter by opening the packaging but not removing the filter. Set aside.
- Using a 5 ml syringe aspirate (suck up) the lysate from the plate.
- Carefully attach the syringe to the filter. Depress the syringe plunger and collect the filtrate in a 15 ml sterile conical tube.
- If you still have unfiltered lysate remaining on your plate, remove the filter and store it in its plastic packaging to maintain sterility. Aspirate the remaining lysate, reattach the used filter, and filter the remaining lysate, collecting the filtrate in the same sterile conical tube.
- Label the tube appropriately.
D. Pool the lysates.
-
- If you have multiple webbed plates for a single phage sample, the same filter can be used for all plates if it remains sterile and unclogged. However, if it has been contaminated or becomes clogged, use a new filter.
- Combine all of the filtered lysates into the same sterile conical tube.
- Record the final volume of lysate collected.
Results:
After 2-4 hours, lysate was successfully collected.
Conclusions and Next Steps:
Plate the first six plates of lysate and proceed.
Title: Full Plate Titer
Date: 11/3/23 Redo: no Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-8
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
XX.
Conclusions and Next Steps:
XX.
Title: Making Webbed Plates
Date: 11/4/23 Redo: no Sample: 7
Purpose:
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-8
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F. Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
XX.
Conclusions and Next Steps:
XX.
Title: Collecting Plate Lysates
Date: 11/5/23 Redo: no Sample: 7
Purpose: To generate a highly concentrated liquid phage sample
Notes:
A. Identify one or more plates for lysate collection. Photograph and label the plate in notebook.
B. Flood the webbed plate(s).
-
- Apply 8 ml of sterile phage buffer to the webbed plate.
- Let the plate(s) sit at room temperature for 2–4 hours.
- Swirl the phage buffer gently, taking care not to splash.
C. Harvest a plate lysate.
-
- When the incubation time is complete, remove the lid from the plate and place it on the bench. Tilt the plate slightly by placing one edge of the plate on the lid, allowing the lysate to pool to one side.
- Prepare a 0.22 μm filter by opening the packaging but not removing the filter. Set aside.
- Using a 5 ml syringe aspirate (suck up) the lysate from the plate.
- Carefully attach the syringe to the filter. Depress the syringe plunger and collect the filtrate in a 15 ml sterile conical tube.
- If you still have unfiltered lysate remaining on your plate, remove the filter and store it in its plastic packaging to maintain sterility. Aspirate the remaining lysate, reattach the used filter, and filter the remaining lysate, collecting the filtrate in the same sterile conical tube.
- Label the tube appropriately.
D. Pool the lysates.
-
- If you have multiple webbed plates for a single phage sample, the same filter can be used for all plates if it remains sterile and unclogged. However, if it has been contaminated or becomes clogged, use a new filter.
- Combine all of the filtered lysates into the same sterile conical tube.
- Record the final volume of lysate collected.
Results:
After 2-4 hours, lysate was successfully collected.
Conclusions and Next Steps:
Plate the first four plates of lysate and proceed.
Title: Full Plate Titer
Date: 11/5/23 Redo: yes Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-12
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
XX.
Conclusions and Next Steps:
XX.
Title: Full Plate Titer
Date: 11/13/23 Redo: yes Sample: 7
Purpose: To determine the concentration of phage particles in a lysate by using a plaque assay
Notes:
A. Perform Serial Dilution of Low lysate phage sample.
- Arrange the proper number of microcentrifuge tubes in a rack and label them 10-1, 10-2, 10-3,….10-12
- Add 90 μl of phage buffer to each of the tubes.
- Add 10 μl of low volumte lysate to the “10-1” tube and vortex well.
- Make sure to use a clean pipette tip for each transfer and pipette carefully, vortexing your sample well before making each dilution. Otherwise, you will not make accurate 10-fold dilutions.
- Transfer 10 μl of the “10 -1” sample to the “10-2” tube and vortex well. This solution contains 1/100th as many phage particles as your undiluted sample. It can also be referred to as your 1:100 dilution.
- Continue each successive dilution until you get to your last tube.
C. Inoculate the host bacteria with your phage sample.
- Obtain the same number of aliquots of 250 μl host bacterial cultures as you have phage samples. Label the culture tubes accordingly.
- Use a micropipettor and aseptic technique to add 10 μL of direct isolation phage sample to the bacterial culture.
- Mix each inoculated host culture by gently tapping the tube.
Important: Make sure your sample makes contact with the bacteria. When you pipette a volume as small as 10 μl sometimes your sample may stick to the side of the tube. - Let the sample sit undisturbed for 5–10 minutes to allow for attachment.
D. Plate the samples with top agar. You will need 3 ml of molten top agar per sample.
-
- Obtain the same number of agar plates as you have samples.
- Remove a bottle of top agar from the 55 °C bath.
Important: You want to keep the top agar in the 55 °C bath for as long as possible to prevent it from prematurely solidifying on your bench. - For each sample:
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
Important: Try to avoid making or withdrawing bubbles, as they can look like plaques on plates. - Immediately aspirate (suck-up) the mixture back into the pipette and transfer it to the appropriate plate and discard the pipette.
Important: The top agar should not sit in the pipette for more than a few seconds because the agar will begin to solidify. - Gently, but quickly, tilt the plate in multiple directions until the top agar mixture evenly coats the agar plate.
- Using a sterile 5 ml pipette, aseptically transfer 3 ml of top agar to an inoculated host tube (i.e., the tube containing bacterial host and phage sample).
- Repeat this process for each of your samples.
E. The next day check the plates and confirm that your dilutions are valid (i.e., a 10-fold decrease in plaques).
-
-
- Use a plate with 20–200 isolated plaques and count the number of plaques.
-
F/ Calculate the titer in pfu/ml using the formula:
Titer (pfu/ml) = (# pfu/ volume used in μl) x (103 μl/ml) x dilution factor*
Results:
XX.
Conclusions and Next Steps:
XX.
DNA Extraction
Title: Phage DNA Extraction
Date: 11/6/23 Redo: No Sample: 7
Purpose: To isolate genomic DNA from phage.
Notes:
-
- Prepare your bench and assemble your supplies.
- Degrade bacterial DNA/RNA in high-titer phage lysate.
- Aseptically transfer 1 ml of phage lysate into a microcentrifuge tube.
- Wearing gloves and working in the designated area, add 5 µl nuclease mix to the lysate.
Important: The enzymes (RNase in particular) are very stable and can persist and contaminate equipment and supplies throughout the laboratory. Take precautions to keep and use them in the designated area. - Mix gently but thoroughly by repeated inversions—do not vortex!
- Incubate at 37 °C for 10 minutes or room temperature for 30 minutes.
- Remove and discard your gloves before returning to your bench.
- Add 15 µl EDTA to the nuclease-treated lysate and mix gently.
- EDTA will inactivate the nucleases by chelating, or binding, divalent cations required by the nucleases for activity.
- Add 0.5 µl Proteinase K and 50 µl SDS to the nuclease-treated lysate and mix gently. Incubate at 37 °C for 10 minutes.
- Proteinase K is added to degrade the nucleases added in Step 2. SDS stimulates the activity of Proteinase K.
Results:
###.
Conclusions and Next Steps:
###.
Title: Mounting Phage Samples for TEM and Staining with Uranyl Acetate
Date: 11/6/23 Redo: Yes Sample: 7
Purpose: To prepare phage sample for viewing with a transmission electron microscope.
Notes:
-
-
- Prepare phage samples.
- Aseptically transfer 1 ml of your high-titer lysate into a sterile microcentrifuge tube.
- Balance the tube(s) and centrifuge for 1 hour at 4 °C at top speed to concentrate the phage particles at the bottom of the tube.
- Using a micropipettor, carefully remove as much supernatant as possible without disrupting the concentrated phage at the bottom of the tube.
- Add 100 μl of phage buffer and let resuspend at 4 °C for 30 minutes to one hour.
- Proceed with the rest of the protocol immediately to avoid damaging the phage heads.
- Prepare your work area. (This may have been done before class by your instructor.)
- Put on a fresh pair of gloves.
- Cover the designated work area with bench paper or a Kimwipe to create a clean work surface.
- Remove the cover from a 5 x 5 cm piece of parafilm, and place the parafilm into the lid of Petri dish.
- Place a PELCO Tab or small piece of double-sided tape onto the parafilm in the lid of the Petri dish. Expose the adhesive or the tab.
- Using EM forceps, remove a fresh grid from a box of unused grids, touching only the very edge of the grid.
- Place the grid dark-and-shiny side UP, on the edge of the tab or double-sided tape so that only the very edge of the grid (no more than 0.5 mm) is touching the adhesive.
- Mount and stain your phage.
- Using a micropipettor, place 10 μl of lysate onto the grid without touching the tip to the grid itself.
- Allow the phage settle and attach onto the grid for at least 2 minutes, or according to the times in Table 8.1b-1.
- Using a small wedge of filter paper, wick off the excess fluid.
- Rinse the grid two times by the following method:
- Carefully pipette 10 μl of sterile water onto the grid. Allow it to sit for 2 minutes.
- Wick off the water using a fresh wedge of filter paper.
Important: Work quickly and carefully! Do NOT allow the grid to dry out!
- Add 10 μl of 1 % uranyl acetate to the grid.
Important: Uranyl acetate is a very toxic compound. You should wear gloves throughout this procedure and when working in any area where this material has been used. - Let it sit for 2 minutes.
- Wick off excess stain by using a wedge of filter paper. UA staining occurs by leaving a very thin layer of stain dried across the entire grid. You should continue to wick away the stain until the surface of the grid looks like a rainbow oil slick. Then allow the grid to air dry before putting it safely back into the grid box.
- Observe your phage.
- Place your grid in the designated grid box for storage. Be sure to accurately record the location of your grid in the box.
- Transport your samples to your EM facility for imaging.
- Calculate the capsid diameter and tail length relative to the size bar.
- Using a ruler, measure the widest point (edge-to-edge, not vertex-to-vertex) of the capsid and the length of the tail (excluding the capsid and the tail tip). If possible, measure multiple phage heads and tails and average their respective values.
- Measure the length of the size bar with the ruler.
- Using the known and relative lengths of the size bar, calculate the length of the capsid and tail.
- Compare your capsid and tail lengths with those of your classmates.
- Record your findings for entry in Phagesdb according to the protocol Entering Phage into the Actinobacteriophage Database.
- Prepare phage samples.
-
Results:
###.
Conclusions and Next Steps:
###.
Title: DNA Extraction
Date: 11/9/2023 Redo: No Sample: 7
Purpose: Extraction of bacteriophage DNA is necessary to amplify and further characterize the sample.
Notes:
This is a two-day process.
Day One:
1. Gently mix your HVL, then aliquot 5mL of your lysate into a 15 mL conical tube. Add 20 uL of nuclease.
2. Once nuclease has been added, gently invert the tube and incubate at 37°C for 10 min.
3. Aliquot lysate into 5 microfuge tubes, 1mL each.
4. To each tube, add 20 uL of ZnCl2, mix gently by inversion, and incubate at 37°C for 5 min.
5. Centrifuge at 10,000rpm for 1min to pellet the phage.
6. Remove supernatants by aspiration, but try not to disturb the pellet. Discard the liquid-filled pipette tip.
7. Resuspend pellets in 500 uL TES buffer per tube, and incubate at 60°C for 15 min. This will denature the capsids, exposing the DNA, while protecting it from the nuclease activity.
8. Add 1 uL of Proteinase K and mix gently. Incubate at 37°C for 10 min to completely eliminate any residual nuclease activity.
9. Add 60 uL of potassium acetate to each tube. Mix well and leave on ice for 15 min. A white, dense precipitate will form. This precipitate is capsids.
10. Centrifuge at 4°C for 1 min at 12,000rpm to pellet the capsids. KEEP THE SUPERNATANTS containing your DNA, and place them into new microfuge tubes. Discard the tubes with the pellets.
11. Add 500 uL of isopropanol to each of the tubes with the supernatant, mix, and leave on ice overnight (or until the next lab).
Day Two:
12. Centrifuge at top speed for 10 min to pellet DNA, and discard the supernatant into a WASTE tube. It is OK if you do not see a pellet.
13. Add 250 uL of 70% ethanol in each tube, and spin again for 1 min, at top speed. This washes the DNA pellet. Discard supernatants into a WASTE tube.
14. Dry the DNA pellets at room temperature by turning them upside-down onto paper towels, tapping out excess liquid, and leaving them upside-down until the pellets begin to turn clear. The tubes can also be placed in a fume hood or 30⁰C incubator to help with drying. DO NOT RUSH THIS STEP! If not dry enough, you will NOT recover enough DNA!
15. Resuspend the first pellet in 50 uL of nuclease-free water. Then use that solution to resuspend the next pellet.
16. Continue until all 5 pellets have been resuspended in the same 50uL of water.
17. Check DNA concentration and quality (A260:280 and A260:230) with the Nanodrop. Record the DNA concentration and A260:280 and A260:230 ratios in your notebook!!
Results:
XX.
Conclusions and Next Steps:
XX.
Title: Ethanol precipitation of RNA/DNA.
Date: 11/10/23 Redo: No Sample: 7
Purpose: XX.
Notes:
-
-
-
-
- Add: 0.1 vols 3M Sodium acetate
- 2.5-3 vols ice cold 100% ethanol
- Vortex to mix thoroughly
- Precipitate at -20°C for 1 hour or overnight or -80°C 1 hour (overnight will give more precipitation if RNA amount is low)
- Centrifuge at full speed (13000rpm), 4°C for 30 minutes
- Wash pellet twice with 0.5ml ice cold 75% Ethanol, spinning at 4°C for 10 minutes each time
- Take Ethanol out, spin quickly (10s top speed) to remove the trace amount of Ethanol as you can
- Air dry the pellet and resuspend in an appropriate volume of Nuclease free water.
Precipitating small amounts of RNA
Glycogen 20ng per sample may be added to the RNA before precipitation to aid visualization when precipitating small amounts of RNA.
- Add 1ul of a 20mg/ml solution of Glycogen (RNase DNase free)
Reagents
3M Sodium Acetate
Nuclease Free Water Ambion 9937
Glycogen 20mg/ml
-
-
-
Results:
###.
Conclusions and Next Steps:
###.
Title: Entering a Phage into the Actinobacteriophage Database.
Date: 11/12/23 Redo: No Sample: 7
Purpose: To prepare phage sample for viewing with a transmission electron microscope.
Notes:
-
-
-
- Name your phage according to the rules found in the “Phages” dropdown menu and “Naming Rules” on phagesdb.org.
- Log in to phagesdb.org or create an active PhagesDB account by clicking on the “Sign in or Register” button at the bottom of the left column on the page.
- Enter your phage on PhagesDB.
- Once you have logged in to PhagesDB, add a phage by selecting the “Data” dropdown menu and clicking on “Add Phage.”
- Fill in as many fields as possible, paying special attention to the following:
- GPS coordinates: If your GPS fields were not prepopulated by the SEA-PHAGES App, you can find the GPS coordinates by using a web mapping service application. One such service can be found via this link: http://www.heywhatsthat.com/profiler.html OR http://bit.ly/yqKB. Simply drag the map to the location where the environmental sample was found, zoom in as much as possible, and click on the map to obtain the GPS coordinates of that location. The output should be to the right of the map and in the N/W Decimal Degree format.
- Program
- Institution
- Bacterial host
- Name
- Convert the GPS coordinates to the correct format if necessary. For example, the coordinates 40° 26’ 46” N, 79° 57’ 11” W from the iPhone’s “Compass” app will be converted into 40.446111 N, 79.953056 W.
Important: Phages must have GPS coordinates to be submitted to GenBank after sequencing and annotation.
- If you found your phage using an enriched culture, click “Yes” to “From Enriched Soil” or “No” if you used a direct isolation.
- Fill in the Discovery Notes to explain where the sample was collected and the soil conditions, etc.
- Complete the Naming Notes to explain where your phage name came from and why you chose the name.
- Enter the details about your phage’s plaque morphology, including size, turbidity, margins, etc.
- The “Morphotype” of your phage is determined from the TEM data. Details about the different morphotypes can be found in Chapter 8, Viewing Phage Particles by Transmission Electron Microscopy.
- The Cluster, Subcluster, Sequencing Facility and Method, and GenBank Accession Number can be filled in only after a phage’s genome has been sequenced. Therefore, you should not fill in these fields.
- If you know the titer of the sample to be archived at the University of Pittsburgh, enter the titer here (or return later and enter the data).
- Upload unaltered images of your phage where indicated. If you do not have a particular image at this time, you will need to return and add it at a later date.
- Click “Submit” to submit your request to PhagesDB. Your submission will be reviewed and approved by a scientist at the University of Pittsburgh; therefore, your submission will not be visible immediately. Please submit your phage only ONCE!
- Once approved, information is publically available for everyone to see and use.
- Once your submission is accepted, you need to create thumbnails of the images that will be displayed with your phage’s profile.
- From the main PhageDB page select the “Data” dropdown menu and click on “Thumbnails.”
- Type in your phage’s name.
- Read the instructions for “Making Thumbnails for Plaque and EM Pictures for PhagesDB.”
- Upload images that fulfill the requirements in the instructions.
- If you need to modify or add data to your phage’s PhagesDB profile, you can do so at any time by clicking on the “Data” dropdown menu and selecting “Modify Phage.”
- Once your phage is sequenced, the SEA-PHAGES team will add data, such as the phage cluster, the genome sequence, and the type of ends. Finally, when your phage has been annotated, submitted to GenBank, and published, the SEA-PHAGES team will add the GenBank accession number and publication data. Phages MUST have GPS coordinates for GenBank submission. Only phages that have been, or will be, archived at the University of Pittsburgh should be entered into the database.
-
-
Results:
###.
Conclusions and Next Steps:
###.
Characterization
Title: Setting Up Restriction Enzyme Digests
Date: 11/13/2023 Redo: No Sample: 7
Purpose: to cut our phage genome into multiple fragments based on its DNA sequence. .
Notes:
-
-
- Prepare genomic DNA.
- Gently mix your DNA sample by either flicking the closed tube with your finger or vortexing it on low.
- Incubate the tube at 65 °C for 10 minutes, and then quickly place it on ice. Quick spin the tube in a microcentrifuge for less than 1 minute to move all of the liquid to the bottom of the tube.
- Using the concentration of your DNA sample, calculate the volume of DNA sample needed to obtain 0.5 µg of DNA.Example: You want to digest 0.5 µg of your phage DNA. If your DNA sample has a concentration of 125 µg/ml, calculate how many µl of DNA are needed to obtain 0.5 µg:
µl DNA = 0.5 µg (ml/125 µg)(1000 µl/1 ml) = 4 µl
- Set up restriction enzyme digest reactions.
- Set up a reaction in a microcentrifuge tube for each enzyme according to Table 10.1-1. Include a negative control in which no restriction enzyme is added.
Important: Add your phage DNA last to prevent contamination of the enzyme stocks!
- Mix the contents of each tube gently and quick spin the tube in a microcentrifuge for less than 1 minute to move all of the liquid to the bottom of the tube.
- Incubate at 37 °C for up to 1 hour. (Note: Some enzymes may require only 15 minutes, so follow your instructor’s directions.)
- Set up a reaction in a microcentrifuge tube for each enzyme according to Table 10.1-1. Include a negative control in which no restriction enzyme is added.
- Quick spin the tube in a microcentrifuge for less than 1 minute to move all of the liquid to the bottom of the tube. Store at -20 °C until ready to use.
- To visualize your digested phage DNA, follow the protocols for Casting Agarose Gels (10.2) and Analyzing Restriction Enzyme Gels (10.4).
- Prepare genomic DNA.
-
Results:
###.
Conclusions and Next Steps:
###.